Nuclear Forces
As you may recall from chapter 2 (here), objects with the same electric charge repel each other, and the strength of that repulsive force is strongest when the objects are close together. Protons all have the same charge (positive), and therefore repel each other—especially when they are clustered tightly together in the tiny nucleus of an atom. So what keeps the nucleus from flying apart?
The nucleus of an atom is held together by an extremely powerful but short-range fundamental force called the strong force. The strong force attracts protons together, and it also attracts neutrons. At close range, the strong force is many times more powerful than the electromagnetic force. That’s why it is able to overcome the electromagnetic repulsion between protons. But the strength of the strong force falls away dramatically over small distances. When protons are located just a few times their own width away from each other (still much closer than the width of an atom), the electromagnetic force pushing them apart is stronger. That’s why the nuclei of neighboring atoms don’t get pulled together.
At macroscopic scales, the magnitude of the strong force is practically zero, so we can’t feel this force like we can feel the forces of electromagnetism and gravity. But the effects of this force, when it acts at close range, are dramatic. As we’ll see later in this chapter, it is the force responsible for the energy released in nuclear power plants, atomic bombs, and—most importantly—the sun and other stars.
If the nucleus of an atom is too large, protons located at opposite sides of the nucleus push each other apart (with the electromagnetic force) more strongly than they attract each other (by the strong force). For this reason, atoms cannot contain arbitrarily high numbers of protons. This explains why there are only finitely many chemical elements. Ununoctium (Uuo) has the highest atomic number of any known element: it contains 118 protons. Ununoctium is not found in nature, but a few isotopes of Uuo have been created in high-energy experiments using particle accelerators, which will be discussed later in this chapter. The nucleus of Uuo usually breaks apart in less than a millisecond. Larger atoms would disintegrate even faster, so they would be difficult to detect even if they could be created.
Another fundamental force, called the weak force, also acts at close range inside the nucleus of an atom. As the name suggests, the weak force isn’t nearly as strong as the strong force; in fact, it’s even weaker than the electromagnetic force (but still much stronger than gravity). The weak force plays a role in radioactive decay—a process (or set of processes) that will be discussed on the next page.
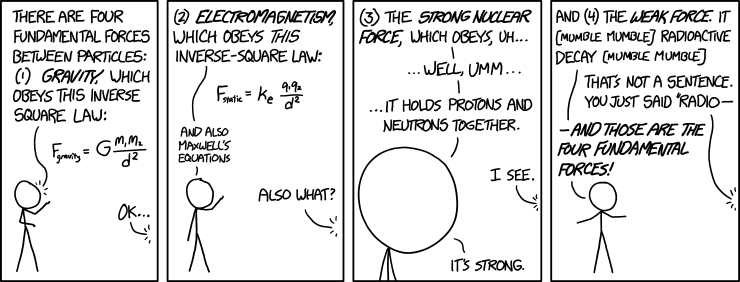
Like gravity and electromagnetism, the strong and weak nuclear forces are fundamental forces, which means that they aren’t explained in terms of more basic forces. The above comic by Randall Munroe pokes fun at the oversimplified and incomplete explanations typically offered to undergraduate physics students when the four fundamental forces are introduced. The fundamental laws governing the strong and weak nuclear forces are the laws of quantum field theory, which will be discussed in chapter 7.